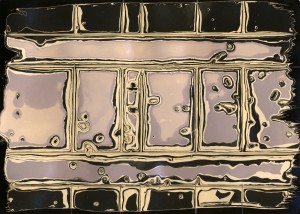
I’ve previously talked about my initial work with the Lumen process and later its combination with the chemigram process. In recent weeks I’ve been experimenting with a some old Kodak Autopositive paper and been amazed by the colour produced. When initially exposed it goes an intense crimson red colour, while this colour is lost during fixing, it is replaced by a fairly intense orangey-yellow colour which is almost as attractive.

Kodak Autopositive paper after initial lumen exposure

Kodak Autopositive paper after fixing lumen exposure
I had heard before that different papers can give wildly different colours when used with the Lumen process, but the papers I’d tried before this were all reasonably similar, so the results from the Kodak paper was a delightful surprise. It did, however, start me wondering what on earth is going on to form such colours from the POV of the chemistry. In order to figure out what might be happening during the Lumen process, it is important to first understand the chemical processes by which normal silver gelatin images are formed…
Traditional Silver Gelatin photographic process
Common photographic emulsion consists of crystals of one of the silver halides (silver bromide, silver chloride or silver iodide), suspended in a gelatin layer. Gelatin is chosen because it is permeable allowing the chemical agents (developer, fixer, toners) to easily access the light sensitive crystals. It also forms a good colloid ensuring an even spread of crystals across the substrate (paper).
Emulsion manufacture
The emulsion is formed by taking silver nitrate and mixing in a gelatin solution that contains halides (potassium bromide, sodium chloride, or potassium iodide). The silver nitrate reacts readily with the halides, the silver atoms combining with the halogen atoms, while the salt combines with the nitrate. Taking potassium bromide as the example halide, the reaction would be
AgNO3 + KBr => AgBr + KNO3
The silver halide molecules form light sensitive crystals, the size & shape of which determine the “grain” of the emulsion. Other active molecules in the gelatin also react with the silver and result in flaws in the crystal lattice. It is these flaws which in fact lead to the photosensitivity of the silver halide.
A number of other chemicals are added which will influence the chemical reaction, the formation of the crystals and thus the characteristics of the emulsion when later exposed to light. A pH buffer will influence the speed of the crystal forming reaction, a crystal habit former will influence the shape of the crystals, a ripener will encourage the formation of crystals, restrainer prevents the reaction taking place too quickly, surfactants lower the surface tension between the liquids to encourage mixing, a defoamer will hinder the formation of foam, stabilizers will prevent other undesirable chemical reactions taking place, biocides will kill any biological impurities in the mixture. While the vast majority of photographic paper emulsions are based on Silver Bromide, the choice of other chemicals used during the manufacturing process may vary wildly across manufacturers or product lines.
The emulsions are usually washed to remove reaction byproducts, specifically the salts and nitrates. Further additives will also be included to control the sensitivity of the emulsion. Usually paper is designed to only be sensitive to blue and green wavelengths of light, allowing use of a red safelight in the darkroom. There are some variations in the choice of sensitive wavelengths, hence the need to use special safelight colours with some papers. There are also panchromatic emulsions which are sensitive to all wavelengths and thus must be used in complete darkness with no safelight. Kodak Panalure is an example of the latter.
Exposure
As mentioned earlier, the silver halide crystals in the emulsion contain small defects leading to gaps in the lattice, as well as mobile silver ions. When a photon of light is incident on the silver halide crystals, it can liberate an electron from the halide. The electron migrates to an sensitivity site where it combines with a mobile silver ion to form a metallic silver atom. The halide atoms are released from the crystal and will collect in the emulsion. As further photons are incident on the crystal, the number of metallic silver atoms grows. These silver atoms form the so called “latent” image in the emulsion, that will be developed into the final image
Development
The goal of development is to intensify the latent image by many orders of magnitude – as much as 109. IOW the few 100 silver atoms in the latent image need to be turned into many 100’s of millions of silver atoms, becoming easily visible. This is achieved through a chemical reaction that converts more of the silver halide crystals into metallic silver atoms. The metallic silver atoms are opaque & non-reflective to light, producing the blacks in the image while the paper of course provides the whites. The key characteristic of the chemical(s) used as the developer is that it should have a much stronger effect on crystals which contain the latent metallic silver atoms after exposure. A developer which affected all crystals indiscriminately would simply result in fog across the entire emulsion.
The stop bath merely neutralizes the action of the developer chemicals, so won’t be discussed further.
Fixing
Once the desired image has been developed, there will still be plenty of silver halide crystals remaining in the emulsion. These are of course still light sensitive, so if left would cause the image to degrade / fog over time. Thus the goal of fixing is to remove all remaining crystals, leaving only the metallic silver image. Ordinarily a sodium thiosulphate solution is used as the fixer, as athough potassium cyanide is a viable alternative, the latter is significantly more toxic and dangerous to deal with. Again considering silver bromide, the reaction the takes place is
AgBr + 2Na2S2O3 => Na3[Ag(S2O3)2] + NaBr
Both of the molecules produced by the reaction with the fixer are soluable and can thus be removed by washing. Washing also serves to remove any unreacted sodium thiosulphate which, given time, is liable to remove the metallic silver of the print too.
Toning
There are a number of ways in which an image can be toned, with differing chemical techniques. Silver conversion toners work by replacing the metallic silver atoms with silver compounds, which exhibit a particular tone. Many of the compounds are in fact more stable than metallic silver atoms, so this can improve the archival quality of the image. Colour coupler toners work by coating the metallic silver atoms with a colour dye. Dye toners are similar, but they coat the emulsion as a whole rather than just the silver atoms. As the name suggests, metal replacement toners use a reaction that replaces the silver atoms with atoms or compounds of a completely different metal such as gold, platinum, copper.

Lumen image of flowers & leaves on vintage Ilford FB paper
Lumen image making with Silver Gelatin
With an understanding of the traditional silver gelatin process, it is time to think about what might be going on with Lumen image making. First are some observations of the process in action
- The entire paper is exposed to broad spectrum light immediately
- Prolonged exposure to ultra-violet light is needed to form an image
- After fixing the paper is no longer sensitive to UV light
- Paper which is wet with water will form a more intense image
- Different papers often result in different colour formation
- The image colour will change during fixing
- Old vintage papers often exhibit more extreme colours than modern emulsions
As the paper is exposed to broad spectrum light, essentially all the silver halide crystals will contain a handful of silver atoms (the latent image), so if developer is applied the image will go entirely black. The lumen image could be formed by a reaction with the silver halide or with the silver atoms. We know, however, that after fixer is applied, the paper is no longer sensitive to UV light. Fixer does not affect the silver atoms, only the silver halides. It follows that the lumen image formation must involve the silver halides, and not the metallic silver atoms. As compared to visible light, UV light has higher energy levels and is well known to have an effect on organic molecules and assist in some chemical reactions. Thus it is plausible that UV light triggers a chemical reaction that would otherwise not occur under normal darkroom enlarger exposure.
Silver gelatin emulsions are based on silver-chloride, silver-bromide or silver-chlorobromide crystals. Based on the range of colours shown in lumen prints on flickr or instagrams, that small handful of different types of crystal appear insufficient to produce the wide range of colours seen on different papers. This rather suggests there is a reaction taking place which involves some other molecules present in the emulsion, besides the halide crystals. From the description of the manufacture of emulsions earlier, it is known that there are a wide variety of chemicals involved, beyond the silver nitrate and halides. While attempts are made to wash them out of the emulsion after production, it is likely that chemicals remain, particularly with older papers when the manufacturing process was less well refined. Thus it is plausible that the lumen process involves a chemical reaction with leftover agents from the manufacturing process.
There is a question of why water might act to intensify the image colour, speeding up image formation. Remember that gelatin emulsion is intentionally permeable to allow developer/fixer chemicals to access the silver halide crystals during traditional processing. Water itself is not sensitive to UV light, so unlikely to be chemically involved in the chemical reaction. Most likely is that water is acting as a transport to ensure a fresh supply of the agents of the chemical reaction so it that it can continue rather than fizzle out.
Based on the above information, a guess can be made as to what takes place during lumen image formation.
- Ultra violet light is incident on the silver halide crystals, liberating halides and silver atoms.
- Ultra violet light is incident on unknown compounds in within the emulsion, breaking them down to release further (unknown) atoms/ions/molecules.
- These atoms/ions/molecules react with the silver atoms to form a variety of silver compounds.
- Water improves mobility of the atoms/ions/molecules, ensuring the reaction between the silver atoms and other compounds in the emulsion continues at a reasonably high rate as compared to when the emulsion is dry.
- Different silver compounds naturally exhibit different colours, and so the variety of chemicals left over from the manufacturing process of the emulsion will affect what compounds (and thus colours) can form.
- Each compound will also have different chemical stability. When the image is fixed, some of the silver compounds are broken down again, resulting in the fading in intensity of the lumen image and distinctive colour shifts. The silver compounds which were more stable do not get affected by the fixer and so remain to provide the final image.
It is frustrating that there is a still a giant unknown of just what molecules in the emulsion are reacting with the silver atoms. Understanding this would likely require access to company records about their manufacturing processes, and/or access to xray spectroscopy equipment.
If this broad hypothesis is correct though, it is probably not the age of the paper affects the different colours seen in lumen printing, but rather the process that was used at the time of production that matters. ie the 40 year old kodak autopositive paper that produces intense crimson colours, probably would have had this same effect the if used for the lumen printing the day after it was made vs today. IOW, taking some modern emulsions which exhibit uninteresting lumen results and storing them for 30 years would not have an impact on what kind of colours a lumen print in the future will produce. This potentially makes the old vintage papers all the more valuable to collect today – once they’re gone, they’re gone and the possibilities they have for lumen printing will be lost forever. Off to eBay again….
If anyone knows better about the chemistry of Lumen image making I’d love to hear about it in the comments, as my own knowledge is limited to ancient school chemistry classes and whatever I could find in the internet…

Chemigram combined with Lumen image of flowers & leaves on vintage Ilford FB paper
 The observant will have noticed the timestamp in the top left corner of the image gets mangled. This is an inevitable result of stacking process when there is part of the image which is moving/changing in every single frame. In this case it is no big deal since the timestamp can either be cropped out, cloned out, or replaced with the timestamp from one single frame. In other scenarios this behaviour might actually work to your advantage. For example, consider taking a picture of a building and a person walks through the scene. If they are only present in a relatively small subset of the total captured images (say 5 out of 100), the median calculation will “magically” remove them from the resulting image, since the pixel values the moving person contributes lie far away from the median pixel values.
The observant will have noticed the timestamp in the top left corner of the image gets mangled. This is an inevitable result of stacking process when there is part of the image which is moving/changing in every single frame. In this case it is no big deal since the timestamp can either be cropped out, cloned out, or replaced with the timestamp from one single frame. In other scenarios this behaviour might actually work to your advantage. For example, consider taking a picture of a building and a person walks through the scene. If they are only present in a relatively small subset of the total captured images (say 5 out of 100), the median calculation will “magically” remove them from the resulting image, since the pixel values the moving person contributes lie far away from the median pixel values. So next time you are in a situation where your camera’s high ISO noise performance is not adequate, consider whether you can make use of image stacking to solve the problem in post processing.
So next time you are in a situation where your camera’s high ISO noise performance is not adequate, consider whether you can make use of image stacking to solve the problem in post processing.